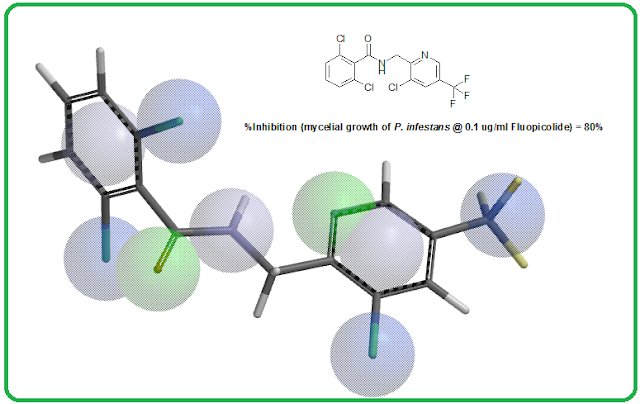
Fluxapyroxad
[3-(difluoromethyl)-1-methyl-N-(3',4',5'-trifluoro-[1,1'-biphenyl]-2-yl)-1H-pyrazole-4-carboxamide]
is a new broad-spectrum fungicide developed and launched by BASF Corporation in
2012. It has both excellent preventative and curative activity through the
inhibition of fungi at several stages of the fungal lifecycle including spore
germination, germ tube growth, appresoria formation and mycelial growth.
Research has demonstrated fluxapyroxad is highly active on several major plant
pathogens from the Ascomycete, Basidiomycete, Deuteromycete and Zygomycete
classes of fungi [1].
 |
| Fluxapyroxad: 2D and 3D Structure |
Fluxapyroxad
inhibits respiration of fungi by blocking production of succinate dehydrogenase
(SDH) and is effective for use on a wide variety of crops, including cereals,
corn, soybean, fruiting vegetables, tuberous and corm vegetables, pome fruits
and stone fruits with excellent crop safety.
Fluxapyroxad
is formulated as an emulsifiable concentrate (EC) or suspension concentrate
(SC) and is foliar appield or used as a seed treatement. As with many other succinate
dehydrogenase inhibitors (SDHI), Fluxapyroxad can be applied in different crops
to prevent a broad range of fungal plant diseases, and is especially
efficacious against leaf spot diseases caused by Ascomycetes species. The compound is reported to have a favorable
toxicological and ecotoxicological profile. The active ingredient trade name
for fluxapyroxad is Xemium® Fungicide. EPA registration is expected in 2012.
Mechanism for Activity
Succinate
dehydrogenase (SDH, complex II) or succinate:ubiquinone oxidoreductase (SQR) is
an enzyme complex, bound to the inner mitochondrial membrane of mammalian
mitochondria and many bacterial cells. SDH is the only enzyme involved in both
respiratory chain and tricarboxylic acid (TCA) or Krebs cycle. In the inner
mitochondrial membrane, SDH catalyzes the oxidation of succinate to fumarate,
coupled with the reduction of ubiquinone to ubiquinol.
In
agrochemical research, SDH was identified as a significant target for
structurally diverse fungicides and acaricides. The fungicidal effect of nearly
all SDH inhibitors relies on the disruption of the TCA cycle.
Fluxapyroxad
is an inhibitor of the mitochondrial succinate-dehydrogenase (SDH). It
economically controls important diseases of the classes Basidiomycetes, Ascomycetes
and Deuteromycetes. After being
applied to the crop, the molecule is systemically (acropetally) distributed in
the plant. In addition to its preventative and long lasting activities, Fluxapyroxad
also provides high curative activity [1].
Developer
Fluxapyroxad
is the result of BASFs ongoing research on succinate dehydrogenase inhibitors
(SDHIs) having started with benodanil, a carboxamide fungicide introduced in
the early 70s of the last century, which mainly provides control of rusts (Puccinia spp.)
Reported Activities for Fluxapyroxad
Fluxapyroxad
has been tested in more than 20 crops against more than 60 pathogens worldwide
including key cereal diseases, Sclerotinia
spp., Cercospora spp., Botrytis spp., Alternaria spp., Venturia
spp., and Colletotricum spp..
Activities for Fluxapyroxad
-
Summary
Common name: Fluxapyroxad; BAS
70001F; BAS 700F
Trademarks: Xemium
Molecular Formula: C18H12F5N3O
CAS Registry Number: 907204-31-3
CAS Name: 3-(difluoromethyl)-1-methyl-N-(3',4',5'-trifluoro-[1,1'-biphenyl]-2-yl)-1H-pyrazole-4-carboxamide
Molecular Weight: 381.30
SMILES:FC1=C(F)C(F)=CC(C2=CC=CC=C2NC(C3=CN(C)N=C3C(F)F)=O)=C1
InChI Key: SXSGXWCSHSVPGB-UHFFFAOYSA-N
InChI: InChI=1S/C18H12F5N3O/c1-26-8-11(16(25-26)17(22)23)18(27)24-14-5-3-2-4-10(14)9-6-12(19)15(21)13(20)7-9/h2-8,17H,1H3,(H,24,27)
Mechanism of Action: Succinate Dehydrogenase
Inhibitors (SDHIs)
Activity: Fungicides; Treatment of Seeds
Status: Launched 2012
Chemical Class: Amides; Small-molecules;
Benzene containing; Ethyl 3-(difluoromethyl)-1- methyl-1H-pyrazole-4-carboxylate
(DFMMP) derivatives; Flouro containing; Difluoro molecules; Trifluoro molecules;
Pentafluoro molecules; Anilines
Originator: BASF Cooperation